Abstract
Background: Systemic mastocytosis (SM) is a clonal hematologic neoplasm, which leads to proliferation and accumulation of mast cells (MCs) in multiple body organs (the skin, bone marrow [BM], spleen, liver, and gastrointestinal tract) causing end organ damage. The KIT D816V mutation is identified in up to 95% of patients and is therefore a major therapeutic target in advanced SM (AdvSM) which consists of aggressive SM, mast cell leukemia, and SM with an associated hematologic neoplasm (SM-AHN). SM-AHN is associated with a high degree of genetic heterogeneity and some patients with high risk AHNs require the use of AHN-directed therapy, such as hypomethylating agents (HMAs). The combination of a highly selective KIT D816V inhibitor with an HMA represents an opportunity to improve response by inhibiting both MC and AHN clones in high-risk patients. AZURE, an international phase 1/2, open-label, 2-arm study, will evaluate the safety and efficacy of BLU-263, an orally administered and selective inhibitor of KIT D816V in patients with AdvSM, both as monotherapy as well as in combination with azacitidine.
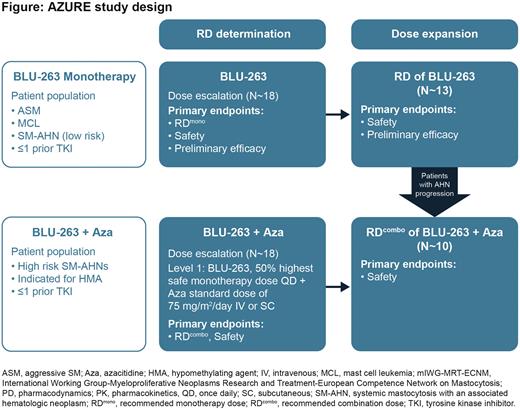
Study design and methods: Approximately 18 patients will initially be treated with escalating BLU-263 monotherapy doses to determine the recommended dose (RD) of BLU-263 (RDmono). This will be followed by dose expansion with an additional 13 patients to further characterize the safety and preliminary efficacy of BLU-263 in AdvSM. Once an initial BLU-263 monotherapy dose has been demonstrated to be safe, patients may begin enrollment in the combination arm, where approximately 18 patients will receive standard azacitidine at doses of 75 mg/m2/day on days 1-7 of each 28-day cycle, and BLU-263 starting at 50% of the monotherapy dose to determine the RDcombo. After RDcombo determination, approximately 10 additional patients will be enrolled into a safety expansion cohort. Dose escalation of monotherapy and combination therapy will follow the rules of Bayesian optimal interval design. Key eligibility criteria include age ≥18 years and Eastern Cooperative Oncology Group performance status 0-3. Eligible patients must have a centrally confirmed pathologic diagnosis of AdvSM per World Health Organization criteria. Patients eligible for the combination therapy include those with chronic myelomonocytic leukemia-2; high- or very high-risk myelodysplastic syndrome (MDS) per International Prognostic Scoring System for Myelodysplastic Syndromes-Revised (IPSS-R); accelerated phase myeloproliferative neoplasm; MDS with excessive blasts-2; complex karyotype/mutational profile; or patients with a hematologic neoplasm and strong rationale for combination treatment following consultation with the sponsor and response assessment committee where needed. Primary endpoints include determination of the RD and safety profile of BLU-263 monotherapy and BLU-263 + azacitidine combination therapy, as well as efficacy according to established response criteria in AdvSM. Secondary endpoints include pharmacokinetic parameters of BLU-263 and BLU-263 + azacitidine, overall response rate, time to response, duration of response, progression-free survival, and the proportion of patients pursuing allogeneic hematopoietic stem cell transplantation.
Disclosures
DeAngelo:AbbVie, Blueprint Medicines Corporation, GlycoMimetics, and Novartis: Research Funding; AbbVie, Amgen, Autolus, Blueprint Medicines Corporation, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Reiter:Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. George:Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; ARUP Laboratories: Other: Associated; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cogent Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees. Radia:Blueprint Medicines Corporation: Membership on an entity's Board of Directors or advisory committees, Other: Educational events, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Cogent Biosciences: Other: Steering committee member for APEX study. Devlin:Blueprint Medicines Corporation: Current Employment, Current equity holder in private company. Dimitrijević:Blueprint Medicines (Switzerland) GmbH: Current Employment, Current equity holder in publicly-traded company. Rinne:Blueprint Medicines Corporation: Current Employment, Current equity holder in private company. Scherber:Blueprint Medicines Corporation: Current Employment, Current equity holder in private company. Gotlib:Cogent Biosciences: Honoraria, Other: Chair for the Eligibility and Central Response Review Committee, Research Funding; Deciphera: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Co-chair of the Study Steering Committee, Research Funding; Blueprint Medicines Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Chair of the Response Adjudication Committee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal